
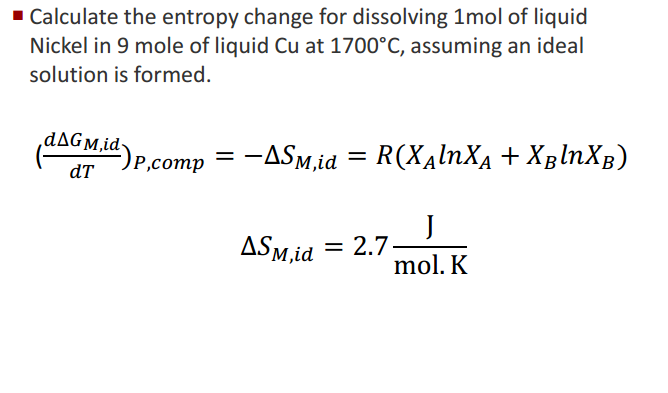
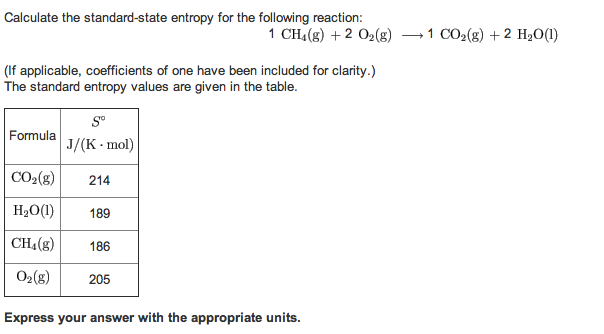
ENTROPY CHANGE CALCULATOR HOW TO
How to calculate Entropy change for Isochoric Process given Temperature using this online calculator? To use this online calculator for Entropy change for Isochoric Process given Temperature, enter Mass of Gas (m gas), Molar Specific Heat Capacity at Constant Volume (C v), Final Temperature (T f) & Initial Temperature (T i) and hit the calculate button. Entropy Change Constant Volume is denoted by ΔS CV symbol. You just have to work out the expression for dS and integrate from T1 to T2 to get ΔS body.How to Calculate Entropy change for Isochoric Process given Temperature?Įntropy change for Isochoric Process given Temperature calculator uses Entropy Change Constant Volume = Mass of Gas* Molar Specific Heat Capacity at Constant Volume* ln( Final Temperature/ Initial Temperature) to calculate the Entropy Change Constant Volume, Entropy change for Isochoric Process given Temperature is defined as the change in the state of disorder of a thermodynamic system that is associated with the conversion of heat or enthalpy into work. Each step would result in a heat flow dQ = mCdT.
ENTROPY CHANGE CALCULATOR SERIES
A reversible process for this would be to successively put it in contact in series with an arbitrarily large number of heat baths - each of which are at an infinitesimally higher temperature than the body - until it reaches T2. The solid body changes temperature from T1 to T2. The reversible path would simply be a reversible isothermal heat flow out of the heat bath of Q, where Q is the amount of heat flow into the solid body. Here you are assuming that the bath has an arbitrarily large heat capacity so its temperature before and after the process is the same. Since the actual process was NOT reversible, the reversible paths will not be the same path for each component. You just have to find a reversible path for each component (ie the solid body and the bath). The addition of a sodium ion to a chloride ion to form sodium chloride is an example of a reaction you can calculate this way. If you know these quantities, use the following formula to work out the overall change: H Hproducts Hreactants. the direction of heat flow) by making an infinitesimal change in conditions.īut to find the change in entropy of each body, you only look at the initial and final states - you do not care about the process that occurred in going from the initial to final states. The most basic way to calculate enthalpy change uses the enthalpy of the products and the reactants.
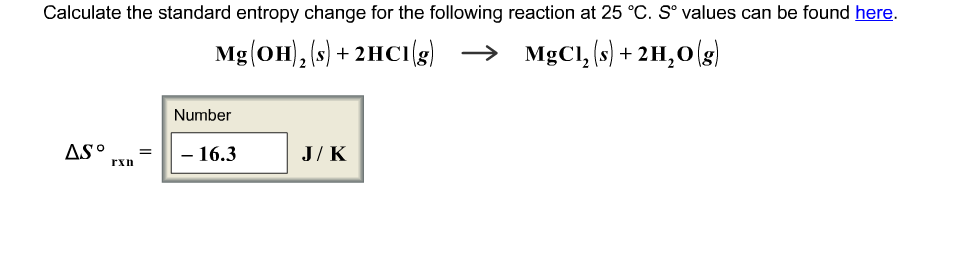
The process is not reversible because you cannot change the direction of the process (ie.
I try to solve this problem by constructing 1 isotherm and 1 adiabat to replace original isochoric path and find out that dS actually = dQ / T = Cv/T dT ,Īlthough i know where this come from ( dS = dQ /T) from maths wayīut I think i misunderstand some physical conceptĬan someone explain why dS = dQ/T, even when this process is not reversible in some physical way? The equation is as follows: S Q/T For a reversible thermodynamic process, entropy can be expressed in calculus as an integral from the initial state of a process to its final state that is dQ/T.
ENTROPY CHANGE CALCULATOR FREE
There comes my question, we find out that this isochoric process is irreversibleīut when we use the equation dS = dQ/T, we could only use it in reversible process. Calculation of Entropy In an isothermal reaction, the entropy change is defined as: S the change in heat (Q) divided by absolute temperature or T. S is entropy Gibbs free energy is a state function. So total entropy is not zero when T2 and T1 has a finite change. This online calculator calculates information gain, the change in information entropy from a prior state to a state that takes some information as given The online calculator below parses the set of training examples, then computes the information gain for each attribute/feature. Then i compute the entropy change of heat body in isochoric system = nR ln(T2/T1)Īnd entropy change of heat bath = - Cv(T2-T1)/T2 ( i read these from notes actually but i am confused with the reversibility in it)īut I remember that we have proved beforeĭS >= dQ/T for all process, and equality hold when process is reversible(like isotherm and adiabats) We could calculate its entropy change(heat body) from dS = Cv/T dTĪnd entropy change of heat bath from dS = dQ / T2, using dQ = Cv dT in 1st equation In an isochoric process ( a solid heat body with T1 temp. This free online devise entropy calculator is helpful to find the change in entropy for a reaction, change in Gibbs free energy and change in entropy of.


 0 kommentar(er)
0 kommentar(er)
